-
background
Cardiovascular disease is the most common cause of death while on the kidney transplant waitlist and after transplantation. Current standard care involves screening for coronary artery disease prior to waitlist entry, then every 1-2 years, according to perceived risk, until transplanted. The aim of screening is two-fold.
Firstly, to identify patients with asymptomatic coronary disease to enable either correction, by bypass surgery or angioplasty, or removal of the patient from the waitlist, with the ultimate aim of preventing premature cardiovascular mortality at the time of, or soon after kidney transplantation.
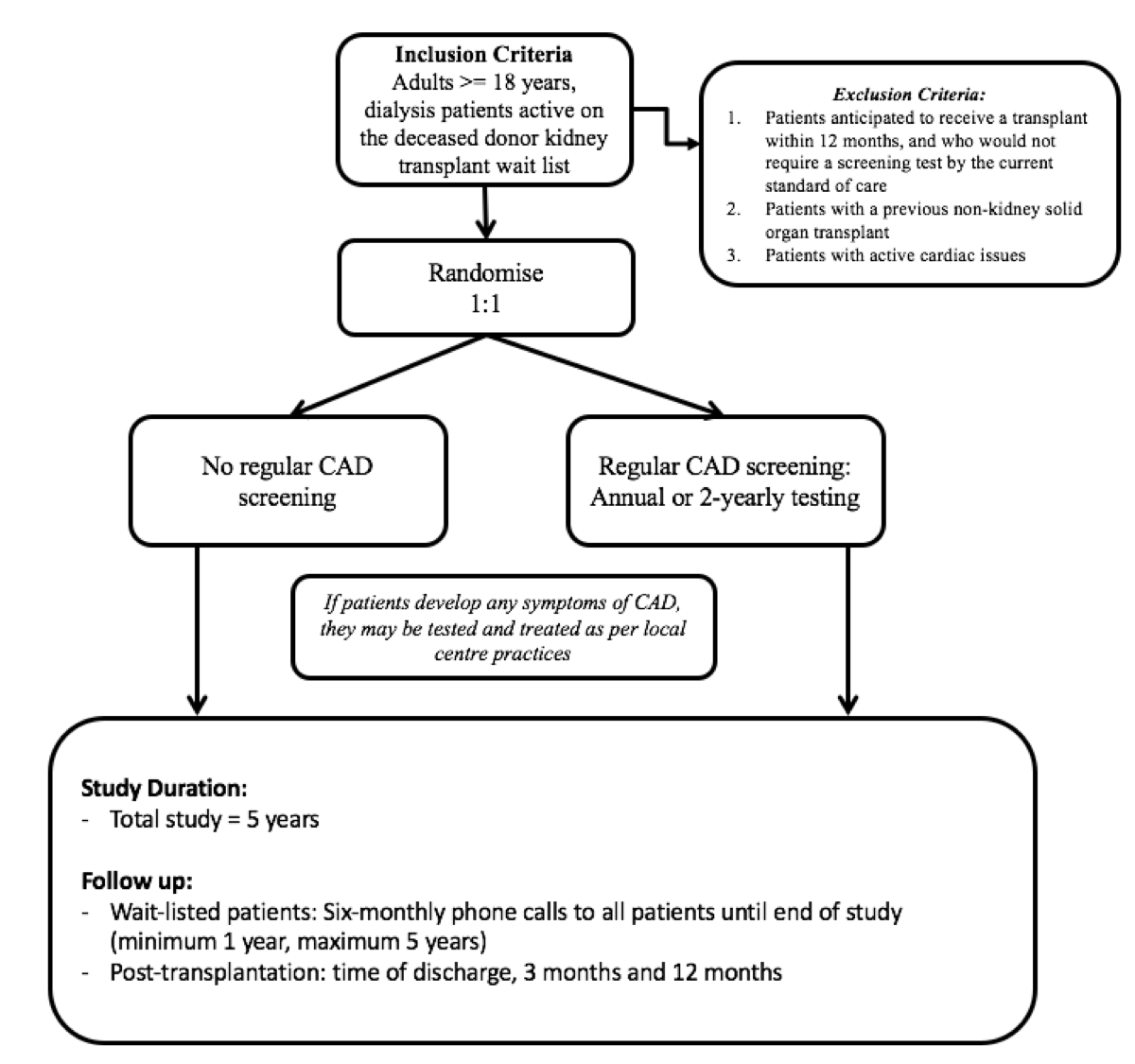
Secondly, from a societal perspective, to prevent misdirection of scarce donor organs into recipients who experience early mortality. This current screening strategy is not evidence based, has substantial known and potential harms, and is very costly. Two major issues of uncertainty require addressing in sequence: (1) whether to periodically screen asymptomatic waitlisted patients for occult coronary artery disease; and (2) whether to revascularise coronary stenoses in asymptomatic patients prior to transplantation. The CARSK study seeks to address the first of these 2 issues.
-
study details
Study design
CARSK is an investigator-initiatied, multicentre, non-inferiority, 2-parallel-arm randomised trial.
Study objectives
CARSK aims to:
1. Test the hypothesis that after screening for wait list entry, no further screening for coronary artery disease (CAD) is non-inferior to the current standard care which is screening all asymptomatic wait-listed patients for CAD at regular intervals.
2. Compare the benefits and costs of not screening versus regular CAD screening from a health system perspective.
Trial interventions:
People randomised to the intervention arm will receive no regular cardiac screening in the absence of symptoms of Coronary Artery Disease.
People randomised to the control arm will receive routine coronary artery disease screening. Additionally all trial participants who develop symptoms or signs of cardiac disease will be investigated and treated as per local protocol.
Study population
We plan to enrol a total of 3,306 patients for the whole trial, 1100 people on the kidney transplant waitlist in Australasia/New Zealand and 2206 in Canada.
-
study endpoints
Primary efficacy endpoint: major adverse cardiac event (MACE), defined as any of the following: cardiovascular death, myocardial infarction, emergency revascularisation, hospitalisation with unstable angina.
Primary safety endpoint; the above MACE endpoint plus complications from cardiac diagnosis or treatment including major bleeding requiring transfusions or hospitalizations, vascular intervention subsequent to cardiac interventions stroke and all-cause death.
Secondary endpoints; death, cardiovascular death, procedure-related death, myocardial infarction, emergency revascularisation, stroke, hospitalisation with unstable angina, hospitalisation with heart failure, hospitalisation with arrhythmia, major bleeding, health-related quality of life (QoL), time off list (including number of temporary suspension and duration of each suspension), cost-effectiveness, incidence of permanent removal from list for cardiac causes; incidence of transplantation and cancellation of transplant due to CAD.
-
inclusion/exclusion criteria
Open
